Physical Properties
- Metals are generally solids at room temperature (with exceptions of mercury, and at times gallium)
- They tend to have high melting and boiling points (because of the strong metallic bonds that keep the metal particles together)
- They are malleable (meaning that they can be pounded into a shape without cracks or breaks)
- This is because the particles are able to move after being pounded by a force without the whole structure breaking apart
- They are also ductile, meaning that their shapes can be changed without losing its strength
- Metals are also good conductors of heat and electricity
Metallic Bonding
- Metals are structured in a lattice, where regularly arranged positive ions are stacked with a freely flowing "sea of electrons" moving around the positive ions
- Because of the free flow of negative ions, electricity is conducted well in metals
- When a metal is pounded and changes shape, it does not completely break apart and is therefore malleable
- This is because even after the positive ions shift in position, they are still bound to the rest of the structure through the attraction with the free flowing electrons

Diagram of a metallic latice (and malleability)
Alloys
- Alloys are mixtures of metals and other elements
- This causes them to have different properties than the original metals (eg. better strength, toughness, resistance...)
- The atoms of alloys also are sized and arranged differently in a way that the layers cannot slide past each other as easily and makes them harder than the regular metal
- Therefore, alloys are often used instead of pure metals
- Some examples of alloys include...
- Brass (a combination of copper and zinc, which is much stronger)
- Iron and Tungsten alloys are very hard and have very high temperature tolerance
- Iron and Chromium or Nickel alloys are more resistant to corrosion (damage)
- Mild steel (a combination of iron and a small amount of carbon) can be bought at a cheap price, used to construct. It is tough and can change shape easily
- Stainless steel (a combination of chromium, iron, nickel, and carbon) has a high resistance to corrosion and is also tough. It's uses are mainly known for cutlery
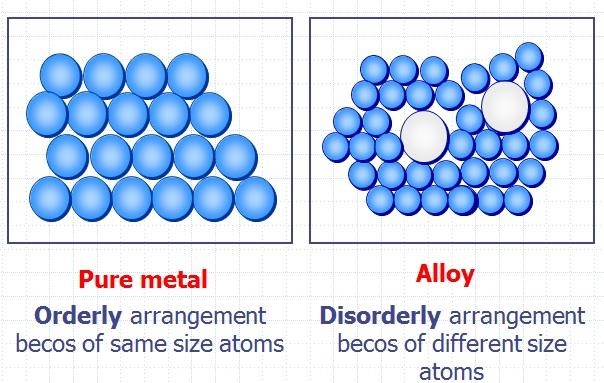
Alloy diagram
Reactivity Series
- The reactivity series shows how reactive each metal is
- This is measured through reactions with water, acids, and oxygen
- The following table will show metal reactions with the most reactive metals at the top and the least reactive metals at the bottom of the table
| Metal | Reaction with Water | Reaction with Acid | Reaction with Oxygen |
| Potassium | Violent reaction | Violent reactionn | Rapid reaction |
| Sodium | Rapid reaction | Violent reaction | Rapid reaction |
| Calcium | Weaker reaction | Vigorous reaction | Quick reaction |
| Magnesium | Mild reaction in water, but burns in steam | Vigorous reaction | Quick reaction |
| Zinc | Does not react with water, reacts with steam | Weak reaction | A reaction occurs |
| Iron | Does not react with water, reacts with steam | Weak reaction | A reaction occurs |
| Hydrogen | Does not react with water | Does not react with acids | A reaction occurs |
| Copper | Does not react with water | Does not react with acids | A reaction occurs |
Reactivity series and forming a postive ion
- Displacement reactions may occur when there are different metals present
- The metal with the higher reactivity will bond with elements that have been bonded with another metal and take them away from the less reactive metals
- For example, with the example CuO + Mg -> Cu +MgO, magnesium takes the O atom from the copper as magnesium has a higher reactivity than copper
- Another case is when an aqueous ion reacts with a metal where the same thing happens
- For example, in the equation Zn (s) + CuSO4(aq) -> ZnSO4(aq) + Cu(s), Zinc "takes" sulfate and forms aqueous zinc sulfate with a remainer of a copper atom
Order of reactivity

Extraction of metals from their ores
- Metals are extracted from ores through taking advantage of the reactivity series
- As metals are finite, they need to be recycled
- Carbon is used to extract metals
- To extract metals higher on the reactivity series than carbon, electrolysis is used
- When electrolysis is used, the process is often expensive due to the large amount of electricity needed
- To extract metals lower on the reactivity series than carbon, heating with carbon is used
- This works as carbon has a higher reactivity, and will isolate the metal through removing what it is bonded with
- This process is cheap because of the cost of carbon
Extraction of Iron from Hematite (with the blast furnance)
- A mixture of iron ore (Fe2O3), coke (C), and limestone (CaCO3) enter the blast furnance
- Coke is an impure carbon, and when a hot air blast is released within the furnance, carbon dioxide is released (C + O2 = CO2)
- Next, carbon dioxide (CO2) and coke (C) form carbon monoxide 2CO
- Afterwards, carbon monoxide gains oxygen from the iron ore to extract the iron (Fe2O3 + 3CO -> 2Fe + 3CO2)
- The iron accumulates and melts at the bottom of the furnance
- Limestone helps remove impurities in the iron ore (CaCO3 -> CaO + CO2). The CaO produced later reacts with SiO2 (an imurity) to form CaSiO3, which accumulates above the iron as slag (impure substance)
Extraction of Aluminium
- Aluminium is higher than carbon on the reactivity series, meaning that it is extracted through electrolysis
- Aluminium is extracted through the ore bauxite which is purified to become aluminium oxide (Al2O3)
- Aluminium is dissolved in molten cryollite to lower the melting point of the aluminium oxide, which means that the process is more cost-efficient and requires less oxygen. However, the cost of the process are still relatively high
- At the cathode, aluminium ions (Al3+) gain 3 electrons to form pure aluminium
- At the anode, oxygen ions (2O2-) lose 4 electrons to form O2
- The carbon anodes may react with the oxygen gas released, causing the carbon anodes to corrode and making replacement of the anode necessary
- Therefore, aluminium is extracted through electrolysis as it is separated from the oxygen atoms
Uses of Metals
Uses of aluminium
- Aluminium will naturally form a alumiium oxide layer by recating with nearby oxygen, allowing it to have a strong resistance tocorrosion
- A thin sheet of aluminium (aluminium foil) is used to contain food as the shape can be easily manipulated and the aluminium does not react to food
- Aluminium is also not very dense, meaning that it is relatively light. However, it is still strong, and is therefore used in different parts of aircraft
Uses of mild steel and stainless steel
- Stainless still does not rust and is resistant to corrosion as well. Also due to its strength, it is used in cutlery and chemical plants
- Mild steel is softer and more malleable, and is therefore used to make the different panels of a car and machine parts
- Zinc is used to make brass with copper, which forms an alloy used in locks, taps, door knobs, and instruments
- Zinc is also used to galvanise steel, which is a process to coat the steel with zinc and prevent rusting
↞Previous Topic Next Topic ↠
