The Periodic Table is a method to arrange elements in a way which we can predict certain trends and properties of these elements
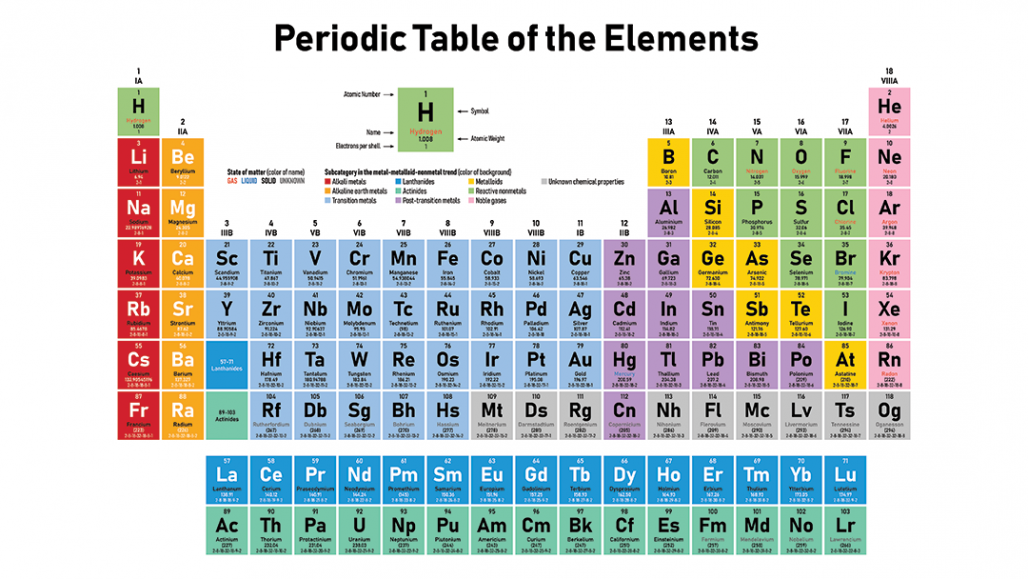
The Periodic Table
Periodic trends
- Metals are found on the left of the periodic table while nonmetals are found on the right
- These are the metals, non-metals, and metalloids in the periodic table
- The group number indicates the number of valence electrons present in the element
- A lower group number means that the element is more metallic, and a higher group numbers means that the element is more non-metallic
Group properties
Alkali Metals, Group I (eg. lithium, sodium, potassium)
- Are relatively soft metals
- They are not very dense
- Their melting points are low
- When you go down the group, the elements get softer and denser
- They react in water very rapidly to produce an alkaline metal hydroxide solution and hydrogen gas
- When you go down the group, the elements react quicker (as the forces between valence electrons and the proton are reduced because of the distance between the valence electrons and the proton). Lithium is the most reactive and francium is the least reactive
- They have 1 valence electron, and therefore is very reactive, as they only need to lose one electron
Halogens, Group VII (eg. chlorine, bromine, iodine)
- Halogens are diatomic non-metals (molecules containing 2 atoms)
- The colour of halogens become darker when going down the group (eg. chlorine is pale green, bromine is reddish brown and iodine is black)
- The elements in this group have 7 valence electrons, and are reactive
- When going down the group, the halogens change in state at room temperature (eg. chlorine is a gas, bromine is a liquid, iodine is a solid)
- The reactivity of halogens increases when going up the group (as the forces between the nucleus and the valence electron to be gained are stronger because of the distance between the proton and the valence electron to be gained). Fluoride is the most reactive halogen, and astatine is the least
Transition elements
- Strong and hard metals that are good conductors of heat and electricity
- They are dense and have high melting points (eg. titanium 1,688°C)
- They form coloured compounds and tend to have several oxidation states (charges). The different oxidation states of the compounds also cause different colours (eg. Fe2+ is green and Fe3+ is orangish brown
- Transition metals are also used as catalysts to speed up reactions (as they have many oxidation states, and can take and lend electrons more easily)
Noble Gases
The noble gases is group VIII or 0
- The group is not reactive (inert) due to the full valence electrons
- This group is very stable and is monatomic (the elements consist of one atom)
- As noble gases already have their full valence electrons, they do not need to react to gain or lose more electrons
Uses of noble gases
- Noble gases are useful in certain cases because it does not react (is inert)
- Helium is used in balloons and air ships because of its low density and because it does not react
- Hydrogen had been used to fill airships before. However, due to its reactive nature to form water vapour with oxygen, the airships had the danger of burning up
- Argon is used in light bulbs so that it does not react and burn up even when the heat within the light bulb is high
↞Previous Topic Next Topic ↠
