- Physical Changes are defined as changes that may change physical properties of an object, but not change the chemical composition
- Chemical Changes are defined as changes which involve chemical reactions and result in the formation of products with different chemical structures than the reactants
- Some chemical reactions are reversible, meaning that the products can once again become the reactants. This includes...
- Hydrated Copper (II) Sulfate⇌ Anhydrous Copper (II) Sulfate:
- Heat is able to evaporate the water from hydrated copper and transform it into Anhydrous Copper (II) Sulfate
- Water can be added through a pipette to anhydrous copper to transform it into Hydrated Copper (II) Sulfate
- Hydrated Cobalt (II) Chloride⇌ Anhydrous Cobalt (II) Chloride:
- Heat and water could be used to reverse the reaction lilke the case above for Copper (II) Sulfate
Elements, Compounds, Mixtures
Differences between elements, compounds and mixtures
- Elements
- Pure substances that are only made up of one type of atom
- Cannot be further broken down chemically
- Compounds
- Comprise of chemically bonded atoms of more than one type
- Can only be chemically separated and not physically separated
- Melt and boil at a fixed point
- Mixtures
- A physical combination of 2 or more substances
- Can be physically separated
- Melt and boil over a range of temperatures
General Differences between metals and non-metals
Metals
- Good conductors of electricity and heat
- Normally solids at room temperature with high boiling and melting points
- Hard and strong
- Malleable (able to be pounded into shape without breaking) and ductile (able to have its shape changed without losing endurance)
- Shiny when polished
- Makes a ringing sound when struck
- Have high densities
- Often form positive ions
- Usually forms basic oxides
Non-metals
- Poor conductors of electricity and heat
- Normally liquids or gases at room temperature with low boiling and melting points
- Hard, but can break easily
- Dull in colour
- Does not make a ringing sound when struck
- Have low densities
- Often form negative ions
- Usually forms acidic oxides
More Definitions
- Solvent: the liquid of the solution which the solute dissolves in
- Solute: the part of the solution that dissolves into the solvent
- Solution: a liquid mixture of a solute dissolved inside a solvent
- Concentration: the ratio of the amount of a component is present in a mixture to the total volume of the mixture
Atomic Structure and the Periodic Table
Structure of an atom
- An atom is made up of...
- a central nucleus made up of protons and electrons
- shells (basically a layer of electrons in a circular orbit) of electrons surrounding the central nucleus
Noble Gas Configuration
- Atoms try to obtain full valence shells
- This means that they try to get the maximum amount of electrons a shell can contain. The maximum amount of electrons the first (or the inner-most) shell can contain is 2. All other shells can contain up to 8 electrons
- Atoms are stable when they have a full outer valence shell
- When atoms are in this stable state, they are said to be in a noble gas structure
Charges and masses of electrons, neutrons and protons
- Electrons, neutrons, and protons all have different charges and masses
- Protons have...
- A relative mass of 1
- A charge of +1
- Neutrons
- A relative mass of 1
- A charge of 0
- Electrons
- A relative mass near to 0
- A charge of -1
Atomic/proton number and nucelon/mass number
- The atomic number of an element displayed on a periodic is equal to the amount of protons in the atom
- The mass number of an element displayed on a periodic table is equal to the amount of nucleons (protons and neutrons). This is because protons neutrons both have a mass of one and electrons have a mass close to 0
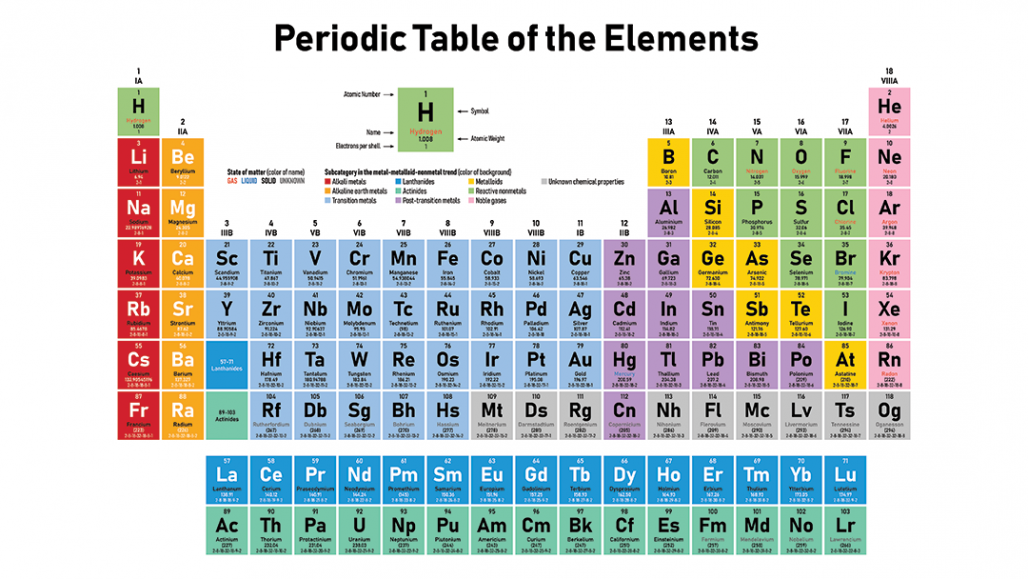
Periodic Table
- The periodic table is a table of atoms which gives you a lot of information. Here is the periodic table provided in the IGCSE
- The Groups are the numbered vertical columns. The first column is group 1, second column is group 2, etc. The groups tell you the amount of valence electrons (electrons in the outer shell) the atoms in the group has. For example, Sodium in Group 1 has one valence electron. Those in the same group normally have similar properties
- The Periods are the rows. They tell you the amount of shells there are the in the atoms in the periods. For example, atoms in period 1 would have 1 shell and atoms in period 2 such as Lithium would have 2 shells
- The elements are arranged by increasing atomic/proton number, meaning that the next atom will always have one more proton than the previous atom
Isotopes
- Isotopes are atoms with the same amount of protons and electrons as a regular atom, but has an irregular amount of neutrons
- This makes the mass of isotopes different
- However, isotopes still have the same properties as regular atoms as they have the same amount of valence electrons (electrons in the outer shell) as a normal atom as valence electrons decide the properties of an atom
Ions and ionic bonds
- Ions are atoms with a charge. Atoms become ions when they lose or gain electrons, resulting in the atom having a charge
- Ionic bonds are formed to achieve a full valence shell or a noble gas structure through losing or gaining atoms
- Metals lose electrons to become positively charged, while non-metals gain them to become negatively charged
- Ionic compounds are held together by the strong charges of the two ions (as positive and negative ions attract)
Group I and VII
- A very common type of ionic bonding is through Groups I and VII
- Group I is a group of metals (with one valence electron in the outermost shell)
- Group VII is a group of non-metals (with seven valence electrons in the outermost shell)
Let's say Sodium (Na) from Group 1 and Chlorine (Cl) were to form an ionic bond
- Sodium (Na) wants to lose its valence electron to have 8 electrons in its outermost shell
- Chlorine (Cl) wants to gain one valence electron to have 8 (7+1) electrons in its outermost shell
- Therefore, Sodium donates its valence electron to Chlorine which receives it. Sodium now has a positive one charge (as it has one more proton than electron) and Chlorine now has a negative one charge (as it has one more electron than proton). They are bound together by their opposite forces
- Dot and cross diagram for NaCl (diagram representing the movement of valence electrons)
- Ionic compounds have a lattice structure, where particles are closely packed. They are arranged with alternating positive and negative ions
- NaCl lattice structure (there is a regular alternating structure of Cl- and Na+)
Molecules and Covalent Bonds
- Covalent bonds are simple bonds between non-metallic atoms
- The non-metallic atoms share electrons to achieve their full valence shell
Here are some examples of covalent bonds (dot-cross diagrams). Click to view the diagrams
- H2 (Hydrogen Gas)
- Cl2 (Chlorine Gas)
- H2O (Water)
- CH4 (Methane)
- NH3 (Ammonia)
- HCl (Hydrochloric Acid)
- N2 (Nitrogen Gas)
- C2H4 (Ethene)
- CH3OH (Methanol)
- CO2 (Carbon dioxide)
Difference between ionic and covalent compounds
- Ionic Compounds
- High melting and boiling points (as compounds are held together by opposition charges)
- Often solid at room temperature
- Usually able to be dissolved in water
- Can conduct electricity when dissolved in water or when melted because of the free flow of ions
- Covalent Compounds
- Low melting and boiling points (Intermolecular forces, meaning the forces in the space between the bonded atoms, are weaker. Therefore atoms in covalent compounds are easier to break)
- Often liquid or gas at room temperature
- Usually unable to be dissolved in water
- All electrons are involved in bonding, meaning that there cannot be free charge of ions and electrons which conduct electricity. Therefore, covalent compounds cannot conduct electricity
Macromolecules
- Macromolecules are molecules with many atoms. For example, carbon is arranged in many different ways that create different macromolecules
- Very big covalent compounds are often extremely hard to break down and have very high melting and boiling points
- Large covalent compounds are normally non-metals
Different forms of carbon
- Carbon can exist in many physical forms. These different forms are called allotropes. The following two allotropes are two examples that are needed for the IGCSE
- Diamond
- Each carbon atom is connected to four more, forming a kind of pyramid shape. It is arranged in a tetrahedral structure
- All covalent bonds are strong and weak intermolecular forces are not present as the atoms are closely packed
- Extremely dense and solid
- Has a very high melting point
- Used in jewelry and cutting
- The tips of cutting tools are coated with diamond to make the tools stronger
Diagram of diamond structure
- Graphite
- Has hexagonal layers of carbon bonds
- One carbon atom connects to 3 others to form hexagons. One electron is left behind for each atom and join a sea of electrons that flow freely between the carbon layers and conduct electricity
- Has a very high melting point
- It is soft and slippery because of the layers that can easily slide off
- The bonds between the layered atoms itself are strong. However, the separate layers are not bonded together and are only held together by weak intermolecular forces, making the layers easy to separate
- Graphite is used pencils and as a lubricant in engines and locks because it is slippery. It is useful because when you rub graphite against a surface, the layers easily come off. For example, when you are writing with a pencil, the graphite layers slide off and leave marks on the paper
- Graphite is also used as an inert (non-reactive) electrode for its ability to conduct electricity
Diagram of graphite structure
Silicon (IV) Oxide or SiO2
- Commonly found as quartz or sand
- Is a tetrahedral structure (like the diamond)
- Every oxygen atom bonds with 2 silicon atoms and every silicon atoms bonds with 4 oxygen atoms
- Very hard and strong
- Has a very high melting point
- Does not dissolve in water and cannot conduct electricity
Diagram of Silicon (IV) Oxide (SiO2) structure
↞Previous Topic Next Topic ↠
