Acids and bases are distinguished by their pH measured by the universal indicator
Acids
- Are substances with a pH ranging from 0-7
- Are sour and corrosive
- Acidity is tested through litmus paper. When blue litmus paper is exposed to acids, the paper turns red
- Acids can neutralise bases to form a salt and water
- When acids react, they lose electrons to gain H+ ions
- The presence of H+ ions makes a substance acidic
- An example of an acid is hydrochloric acid, which completely separates into H+ and Cl- ions
Bases
- Are substances with a pH ranging from 7-14
- Bases that can dissolve in water are called alkali
- Acidity is tested through litmus paper. When red litmus paper is exposed to acids, the paper turns blue
- When alkalis react, they gain electrons to gain OH- ions
- An example of a base is sodium hydroxide, which completely separates into Na+ and OH- ions
The pH scale
- It is a scale from 0 to 14, with 0 being extremely acidic, 14 being extremely basic, and 7 being neutral
- All acids have pHs lower than 7 and all bases have pHs higher than 7
- The lower the pH, the more acidic the substance is, and the higher the pH, the more basic the substance is
Universal Indicator
- The pH level of a substance can be assessed by the universal indicator
- A drop of the universal indicator can be added to a solution which changes its colour
- Each colour represents a certain pH
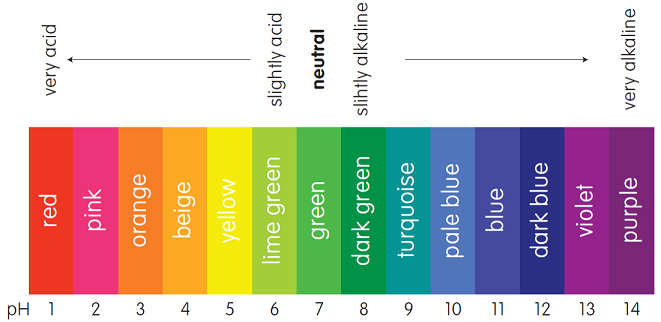
Diagram of the universal indicator
Importance of controlling acidity in soil
- Most plants grow the best at a pH of 5-8
- If the acidity of the soil changes, the pH of the plant may exit this range. This causes a decrease in plant growth and less crop produced
- The acidity of the soil can be affected by acid rain, the excessive use of fertilisers, and excessive breakdown of organic matter by bacteria
- Crushed limestone (a base) is added to the soil to neutralise the soil
- However, if too much limestone is added, the soil will become to basic and harm the plants instead
Types of Oxides
Oxides are compounds which consist of an element bonded with one or more oxygen atoms. They are different types of oxides, which include...
- Basic oxides...
- Consist of a metal atom and oxygen
- React with acids to form a salt and water
- Form a basic solution when dissolved in water
- Acidic oxides...
- Consist of a nonmetal atom and oxygen
- React with bases to form a salt and water
- Form an acidic solution when dissolved in water
- Neutral oxides...
- Oxides that do not react with acids and bases
- Amphoteric oxides...
- Oxides that react with both acids and bases and act as both acidic and basic oxides to form a salt and water
- If the other reactant is a base, the amphoteric oxides act as acidic oxides, and if the other reactant is an acid, the amphoteric oxides act as basic oxides
Preparation of salts
- A salt is a compound that is made up of a positive ion of the base and a negative ion of the acid
- Some salts can be extracted by mining, but some need to be prepared in the lab
- Salts can be insoluble and soluble in water, and different preparation methods are needed depending if the salt is water-soluble
- There are different methods to prepare salts
Filtration
- Used to separate a liquid and insoluble solid
- When the mixture is poured on filter paper, the smaller molecules of the liquid will pass through while the larger solid will not and remain on the paper as residue.
- Method
- Set up the experiment so that filter paper is placed inside of the filter funnel, which should be placed in the hole of a container such as the conical flask in the diagram
- Pour the mixture in the filter funnel
Crystallisation
- Used to separate a solid that has been dissolved into a solution
- Method
- Heat the solution and wait for the solvent to evaporate
- Eventually, the maximum amount of solvent will be dissolved. This is called the saturated solution.
- Dip a glass rod in the solution. When it is saturated, crystals will form on it.
- The solid will become less soluble (less able to dissolved), and the crystals will therefore grow
- The crystals can be collected through filtration
Distillation
- Can separate the solvent and solute in a solution
- Can separate pure liquids from mixtures of liquids
- Method
- Heat the solution to evaporate the pure liquid into a vapour
- The vapour will condense in the condenser, cooling to turn back into a pure liquid in a beaker
- The rest of the mixture will be left behind and therefore separated in the flask containing the original mixture
Precipitation
- Can separate insoluble salt
- Mix soluble salts and water together
- Remove the excess salt by filtering it
- Wash the solution away from the excess salt with distilled water
- Dry the excess salt in an oven, and an insoluble salt will be formed
Depending on whether the salt is insoluble or soluble, you can select one of the methods above
↞Previous Topic Next Topic ↠
